Influence of second-order saddles on reaction mechanisms†
Abstract
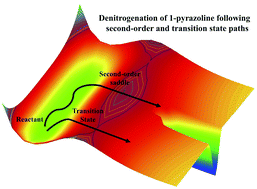
The transition state, a first-order saddle point on the potential energy surface, plays a central role in understanding the mechanism, dynamics, and rate of chemical reactions. However, we recently identified energetically accessible second-order saddles (SOS) in certain reactions and showed that the SOS plays a crucial role in the dynamics of the reactions [Pradhan et al., Phys. Chem. Chem. Phys., 2019, 21, 12837; Rashmi et al., Regul. Chaotic Dyn., 2021, 26, 119]. In the present work, we investigated the role of second-order saddle points on the dynamics of the thermal denitrogenation of 1-pyrazoline using ab initio classical trajectory simulations at the CASSCF(4,4)/6-31+G* level of theory, for total energies of 130, 140, and 150 kcal mol−1 available to the system. In this unimolecular dissociation reaction, the SOS point is 4 kcal mol−1 higher in energy than the synchronous bond-breaking transition state and opens up an additional reaction pathway. We found that the fraction of molecules following the synchronous bond-breaking pathway decreased with an increase in the total available energy in the reaction, accompanied by an increase in the fraction following the asynchronous pathway. To further understand the competition between the transition state and the SOS pathways, we investigated the mechanism of halogen-substituted 1-pyrazolines where the SOS energies are comparable to that of the transition states.
- This article is part of the themed collection: Unimolecular reactions


 Please wait while we load your content...
Please wait while we load your content...