Phenolate-bonded bis(μ-oxido)-bis-copper(iii) intermediates: hydroxylation and dehalogenation reactivities†
Abstract
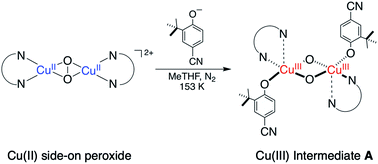
Exogenous phenolate ortho-hydroxylation by copper oxidants formed from dioxygen is generally thought to occur through one of two limiting mechanisms defined by the structure of the active oxidant: an electrophilic μ-η2:η2-peroxo-bis-copper(II) species as found in the oxygenated form of the binuclear copper enzyme tyrosinase (oxyTyr), or an isomeric bis(μ-oxido)-bis-copper(III) species (O) with ligated phenolate(s) as evidenced by most synthetic systems. The characterization of the latter is limited due to their limited thermal stability. This study expands the scope of an O species with ligated phenolate(s) using N,N′-di-tert-butyl-1,3-propanediamine (DBPD), a flexible secondary diamine ligand. Oxygenation of the [(DBPD)Cu(I)]1+ complex at low temperatures (e.g., 153 K) forms a spectroscopically and structurally faithful model to oxyTyr, a side-on peroxide intermediate, which reacts with added phenolates to form a bis(μ-oxido)-bis-copper(III) species with ligated phenolates, designated as an A species. The proposed stoichiometry of A is best understood as possessing 2 rather than 1 bonded phenolate. Thermal decomposition of A results in regiospecific phenolate ortho-hydroxylation with the ortho-substituent as either a C–H or C–X (Cl, Br) group, though the halogen displacement is significantly slower. DFT and experimental studies support an electrophilic attack of an oxide ligand into the π-system of a ligated phenolate. This study supports a hydroxylation mechanism in which O–O bond cleavage of the initially formed peroxide by phenolate ligation, which precedes phenolate aromatic hydroxylation.
- This article is part of the themed collection: Natural and artificial metalloenzymes


 Please wait while we load your content...
Please wait while we load your content...