C-Doped KNbO3 single crystals for enhanced piezocatalytic intermediate water splitting†
Abstract
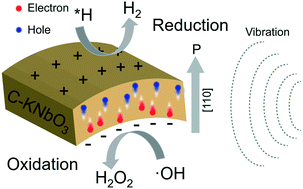
H2O2 is an environmentally friendly chemical for wide water treatments and H2 is regarded as an ideal substitute for traditional fossil energy. The industrial production of H2O2 is via the anthraquinone oxidation process, which, however, consumes extensive energy and produces pollution. Herein, based on the density functional theory (DFT) calculations, we report a green and sustainable piezocatalytic intermediate water splitting (IWS) process to simultaneously obtain H2O2 and H2 using C-doped KNbO3 nano single crystals as catalysts. The KNbO3 catalysts doped with suitable amounts of carbon show an enhanced water splitting rate, which could be attributed to the decreased reaction barriers on the C-KNbO3 surface and improved polarized built-in electric field, as evidenced by DFT simulations and PFM measurements. Remarkably, the piezocatalytic H2 evolution efficiency of 0.5 : 1 C-KNbO3 reaches 524.51 μmol g−1 h−1. Additionally, 0.5 : 1 C-KNbO3 could generate H2 and H2O2 simultaneously through two-electron piezocatalytic IWS reactions. This improved catalytic efficiency is attributed to the promoted piezo-response of KNbO3 after C doping, as revealed by PFM tests.


 Please wait while we load your content...
Please wait while we load your content...