Insights on Pb(ii) retention and immobilization by ferrihydrite in the presence of Al(iii) and oxalic acid†
Abstract
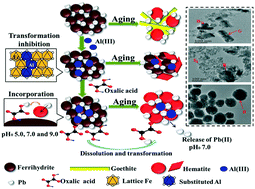
Lead (Pb) is harmful to humans and the natural environment. Unstable ferrihydrite with Pb(II) (Fh-Pb) transforms to more stable iron oxides over time. Al(III) and oxalic acid exist widely in nature, affecting the transformation of Fh-Pb. We herein studied the impacts of Al(III) and oxalic acid on the redistribution of Pb(II) during the Al-containing ferrihydrite–Pb(II) co-precipitate ageing process. Results indicated that Al(III) impeded the transformation of Fh-Pb, while oxalic acid counteracted the inhibition of Al(III) on the Fh-Pb interaction. The coexistence of Al(III) and oxalic acid hampered the release of Pb(II) to the environment under acidic conditions. Under neutral conditions, Al(III) promoted Pb(II) retention without oxalic acid, and oxalic acid caused the release of Pb(II) to the environment. Under alkaline conditions, Al(III) assisted Pb(II) intrusion into the iron oxides in the presence or absence of oxalic acid. This study contributes significantly to understanding the effect of selected impurities and organic substances on the Pb(II) behaviour at various pH values in environmental systems, namely in geological radioactive disposal sites, wastewater ponds and mine sites.


 Please wait while we load your content...
Please wait while we load your content...