Insights into the effect of citric acid on the carbon dot-mediated transport of Cd2+ through saturated porous media†
Abstract
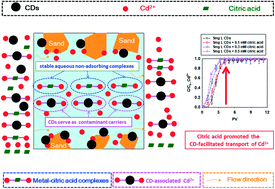
Carbon dots (CDs) are likely to encounter heavy metal ions (i.e., Cd2+) because of their widespread application and inevitable release into the environment, wherein the ubiquitous low-molecular-weight organic acids (LMWOAs) may affect their co-transport behaviors in aquifer systems. Herein, citric acid was used as a typical LMWOA. The effect of citric acid on the CD-mediated transport of Cd2+ through sand columns was investigated. The results demonstrated that CDs could serve as carriers to markedly enhance the transport of Cd2+, which was attributed to the abundant adsorption sites of nanoparticles for Cd2+ and the excellent mobility of CDs. Citric acid promoted the CD-facilitated transport of Cd2+ due to the formation of water-soluble metal–citric acid complexes, the facilitation of the adsorption of Cd2+ on the CDs by citric acid, and the high mobility of the CDs. Interestingly, the enhanced role of citric acid in CD-facilitated transport declined as the pH was raised from 6.0 to 8.0, since the predominant Cd2+ species (e.g., free Cd2+) in the influents preferred to deposit onto sand surfaces at a higher pH. The findings provide meaningful insights into understanding the co-transport of carbon-based nanoparticles and heavy metals affected by the presence of LMWOAs in the natural subsurface environment.


 Please wait while we load your content...
Please wait while we load your content...