Prediction of biphasic separation in CO2 absorption using a molecular surface information-based machine learning model†
Abstract
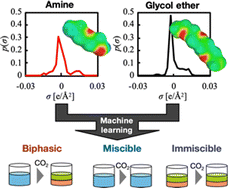
Carbon dioxide capture technologies have become a focus to overcome global warming. Biphasic absorbents are one of the promising approaches for energy-saving CO2 capture processes. These biphasic absorbents are mainly composed of a mixed solvent composed of alkanolamine and organic solvents like glycol ether or alcohol. However, screening experiments of the mixed-solvent absorbents are required to search for biphasic absorbents due to their complicated phase behavior. In this work, we developed a prediction method for the phase states of the mixed-solvent absorbents using a quantum calculation and machine learning models, including random forest, logistic regression, and support vector machine models. There are 61 mixed-solvent absorbents containing alkanolamine/glycol ether or alcohol in the dataset. The machine learning models successfully predicted the phase states of the mixed-solvent absorbents before and after CO2 absorption with accuracies of more than 90%. Furthermore, we analyzed the contributions of explanatory variables for prediction using the learned model. As a result, we found that molecular surface charge of the amine species is more important than those of the other organic solvents to determine the phase behaviors during CO2 absorption.


 Please wait while we load your content...
Please wait while we load your content...