Towards a comprehensive understanding of malathion degradation: comparison of degradation reactions under alkaline and radical conditions†
Abstract
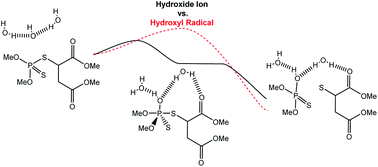
Malathion is a commercially available insecticide that functions by acting as an acetylcholinesterase inhibitor. Of significant concern, if left in the environment, some of the products observed from the degradation of malathion can function as more potent toxins than the parent compound. Accordingly, there are numerous studies revolving around possible degradation strategies to remove malathion from various environmental media. One of the possible approaches is the degradation of malathion by OH˙ radicals which could be produced from both artificial and biological means in the environment. While there is plenty of evidence that OH˙ does in fact degrade malathion, there is little understanding of the underlying mechanism by which OH˙ reacts with malathion. Moreover, it is not known how competitive the radical degradation pathway is with analogous alkaline degradation pathways. Even less is known about the reaction of additional OH˙ radicals with the degradation byproducts themselves. Herein, we demonstrate that OH˙ induced degradation pathways have variable competitiveness with OH− driven degradation pathways and, in some cases, produce quite different reactivity.
- This article is part of the themed collection: Contaminant remediation and fate


 Please wait while we load your content...
Please wait while we load your content...