Cement clinker precursor production in an electrolyser†
Abstract
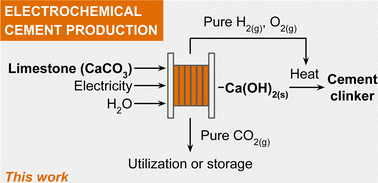
The manufacture of cement is the single largest industrial source of CO2(g) emissions into the atmosphere. We report here an electrochemical flow reactor (electrolyser) that continuously converts limestone (CaCO3(s)) into Ca(OH)2(s) at a high rate of product formation (486 mg h−1 at 100 mA cm−2). The Ca(OH)2(s) product (slaked lime) is a chemical precursor to cement clinker, the main component of Portland cement, and other cement varieties. This three-compartment electrolyser operates with ∼100% current efficiency at a cell voltage of 2.9 V and generates pure O2(g), H2(g), and CO2(g) streams that can be utilized downstream without purification. To demonstrate this feature, we feed the CO2(g) released from limestone directly to a second electrolyser that valorizes CO2(g) into higher value carbon-containing products (e.g., CO). A preliminary life-cycle analysis indicates that the proposed electrochemical process can decrease the amount of CO2 emitted per tonne of cement by 75% and achieve cost-parity with incumbent cement manufacturing processes.


 Please wait while we load your content...
Please wait while we load your content...