High energy density Na-metal batteries enabled by a tailored carbonate-based electrolyte†
Abstract
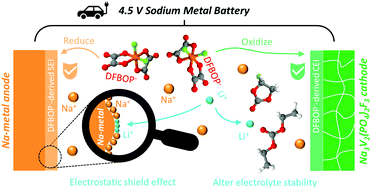
High-voltage sodium metal batteries (SMBs) offer a viable way toward high energy densities. However, they synchronously place severe demands on the electrolyte for the notorious reactivity of Na-metal and the catalytic nature of aggressive high-voltage chemistries. Here, we fabricate a tailored carbonate-based electrolyte involving lithium difluorobis(oxalato) phosphate (LiDFBOP) as a multifunctional additive, where DFBOP− anions can generate stable and robust interphases on both the anode and cathode. Meanwhile, Li+-ions can take part in the solvation structure to regulate the electrolyte stability as well as resist dendritic deposition via electrostatic shielding. Such optimization effectively realizes high coulombic efficiency (98.6%) and prolonged life (2600 h) of Na plating/stripping together with the upgraded reversibility of the Na3V2(PO4)2F3 cathode. Moreover, the assembled 4.5 V Na||Na3V2(PO4)2F3 SMB achieves impressive cycling stability with 90% capacity retention after 220 cycles and a high energy density of 295 W h kg−1 with limited Na. The proposed electrolyte strategy can shed light on further optimization for high-energy sodium metal chemistries.


 Please wait while we load your content...
Please wait while we load your content...