Chemical and structural evolutions of Li–Mn-rich layered electrodes at different current densities†
Abstract
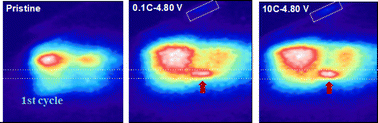
Although the two active redox centers in Li-rich cathodes, including the anionic and cationic contributions, can enable Li-ion batteries to achieve outstanding specific energy, their behaviors at different current densities have not been clarified. Here, we provide a comparative study of transition metals (TMs) and oxygen redox activities by directly accessing their oxidation states in Li-rich materials operated at very different current rates. Our data reveal that the oxidation of oxygen in the near-surface region is at the same level for electrodes cycled with a wide range of current rates, indicating a reaction gradient of lattice oxygen redox reactions. The oxidation process of lattice oxygen is found to be dynamically compatible with that of the TMs. Combining the results of first principles calculations and complementary experimental findings, we propose a detailed mechanism of structural distortion from octahedral Li to tetrahedral Li and the role of oxygen vacancy in Li+ diffusion. It is found that fast delithiation occurring at high current densities can easily cause local structural transformation, leading to a limited Li+ diffusion rate and consequently suppressing rate capability.


 Please wait while we load your content...
Please wait while we load your content...