Reconstruction-induced NiCu-based catalysts towards paired electrochemical refining†
Abstract
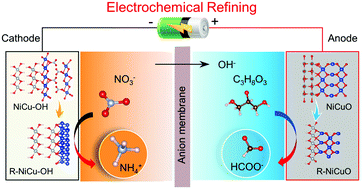
A paired electrochemical refinery toward the cathodic nitrate reduction reaction (NO3RR) and anodic glycerol oxidation reaction (GOR) driven by renewable electricity could generate high value-added ammonia and formic acid simultaneously. However, both cathode and anode electrocatalysts often suffer from low efficiency and unclear reconstruction. Herein, we prepare a non-noble NiCu–OH nanocomposite precatalyst composed of crystalline Cu2(OH)3(NO3) and amorphous Ni(OH)2, which can be reconstructed into high-activity and stable cathodic NO3RR and anodic GOR electrocatalysts under operating conditions. Various in situ and ex situ experiments demonstrate that NiCu–OH could convert into active Cu nanoparticles coupled with amorphous Ni(OH)2 under cathodic NO3RR conditions. Meanwhile, under anodic GOR conditions, NiCu–OH-derived bimetal oxide (NiCuO) changes into active NiOOH species composited with Cu vacancy-rich CuO. Furthermore, an actual paired electrochemical refinery with the reconstructed NiCu-based catalysts toward the NO3RR and GOR shows a low potential of 1.37 V at a current density of 100 mA cm−2 to continuously produce ammonium and formate. This study provides a new paradigm for the design of an electrochemical refinery with reconstructed non-noble electrocatalysts toward chemical upgradation.


 Please wait while we load your content...
Please wait while we load your content...