Boosting water decomposition by sulfur vacancies for efficient CO2 photoreduction†
Abstract
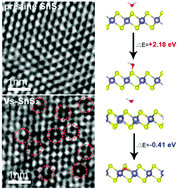
The water oxidation reaction (i.e., OER) is one of the most challenging reaction steps in the overall photocatalytic CO2 reduction, especially on metal sulfide photocatalysts. Herein, we first demonstrate that well-designed S-vacancies on SnS2 atomic thin layers (denoted VS-SnS2) can directly enhance water oxidation in the overall photocatalytic CO2 reduction by promoting the formation of molecular O2. As a result, an average 8.2 times higher CO2 photoreduction efficiency (CO evolution rate of 25.71 μmol g−1 h−1) was obtained on the champion VS-SnS2 (23.07%) catalyst than that on the pristine SnS2 catalyst (CO evolution rate of 3.14 μmol g−1 h−1) upon white light illumination. Two types of water decompositions (1660 cm−1/1620 cm−1) that vary with the S-vacancy concentration were observed by in situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS), demonstrating the key role of the S-vacancy-induced water decomposition configuration (1660 cm−1). Kinetic analysis confirms that the water decomposition reaction is the rate-determining step in the overall CO2 photoreduction and was indeed accelerated on S-vacancy sites (k1660 = −0.033/k1620 = −0.021). Density functional theory (DFT) calculations suggest that S-vacancies facilitate the OER by lowering the energy barriers of intermediate transformations.


 Please wait while we load your content...
Please wait while we load your content...