A thermochemical study of iron aluminate-based materials: a preferred class for isothermal water splitting†
Abstract
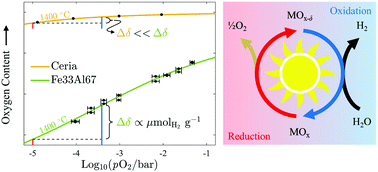
The use of hydrogen as a renewable fuel has been stymied by our inability to produce it cleanly and economically. The conventional solar thermochemical approach considers a two-step redox cycle with benchmark ceria or a perovskite in a temperature swing configuration, where reduction occurs at a temperature much higher than oxidation. Isothermal redox cycling is feasible and avoids the solid–solid heat recuperation and material stability challenges associated with temperature swing; yet, it has long been thought to be inefficient due to the thermodynamic unfavorability of operating the exothermic oxidation reaction at higher temperatures. Here, we show that this setback can be overcome with iron aluminate-based spinel solid solutions that preferentially exhibit large changes in oxygen content within the range of oxygen partial pressures expected in large-scale systems. We explain the experimental results with a defect model that assigns cation – not oxygen – vacancies as the predominant point defect responsible for their superior water-splitting ability. When operated isothermally at 1400 °C, the iron aluminate-based materials demonstrate a capacity for hydrogen production greater than 500 μmol g−1 and, as a result, remain viable even under high conversion conditions (i.e., pO2 < 500 : 1 H2O : H2), exceeding the hydrogen yields of ceria and two attractive perovskite candidates following a 400 °C (or less) temperature swing. Isothermal water splitting using iron aluminate-based materials opens the door for more simple, robust, and efficient production of renewable hydrogen.


 Please wait while we load your content...
Please wait while we load your content...