Decomposition mechanism of α-alkoxyalkyl-hydroperoxides in the liquid phase: temperature dependent kinetics and theoretical calculations†
Abstract
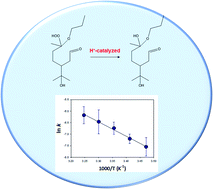
Organic hydroperoxides (ROOHs) play key roles in the atmosphere as a reactive intermediate species. Due to the low volatility and high hydrophilicity, ROOHs are expected to reside in atmospheric condensed phases such as aerosols, fogs, and cloud droplets. The decomposition mechanisms of ROOHs in the liquid phase are, however, still poorly understood. Here we report a temperature-dependent kinetics and theoretical calculation study of the aqueous-phase decompositions of C12 or C13 α-alkoxyalkyl-hydroperoxides (α-AHs) derived from ozonolysis of α-terpineol in the presence of 1-propanol, 2-propanol, and ethanol. We found that the temporal profiles of α-AH signals, detected as chloride-adducts by negative ion electrospray mass spectrometry, showed single-exponential decay, and the derived first-order rate coefficient k for α-AH decomposition increased as temperature increased, e.g., k(288 K) = (5.3 ± 0.2) × 10−4 s−1, k(298 K) = (1.2 ± 0.3) × 10−3 s−1, k(308 K) = (2.1 ± 1.4) × 10−3 s−1 for C13 α-AHs derived from the reaction of α-terpineol Criegee intermediates with 1-propanol in the solution at pH 4.5. Arrhenius plot analysis yielded an activation energy (Ea) of 12.3 ± 0.6 kcal mol−1. Ea of 18.7 ± 0.3 and 13.8 ± 0.9 kcal mol−1 were also obtained for the decomposition of α-AHs (at pH 4.5) derived from the reaction of α-terpineol Criegee intermediates with 2-propanol and with ethanol, respectively. Based on the theoretical kinetic and thermodynamic calculations, we propose that a proton-catalyzed mechanism plays a central role in the decomposition of these α-AHs in acidic aqueous organic media, while water molecules may also participate in the decomposition pathways and affect the kinetics. The decomposition of α-AHs could act as a source of H2O2 and multifunctionalized species in atmospheric condensed phases.
- This article is part of the themed collections: ES: Atmospheres Hot Papers and SDG13: Climate Action- chemistry of greenhouse gases, 2022


 Please wait while we load your content...
Please wait while we load your content...