Direct arylation reaction catalyzed by a PEPPSI-type palladium complex with an amido-functionalized triazole-based mesoionic carbene ligand†
Abstract
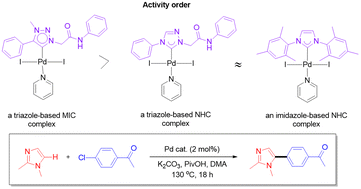
Ligand precursors for amido/amidate-functionalized triazole-based MIC ligands were synthesized. An initial theoretical calculation confirmed that triazole-based MIC ligands were promising ligands in terms of their σ-donating and π-acidic properties. Based on these ligand precursors, three different types of palladium complexes were successfully obtained, namely (1) a PEPPSI-type MIC complex, (2) a complex containing both a bidentate ligand of a MIC and an amidate donor and a mondentate NHC derived from nitron, and (3) a complex featuring a tridentate ligand of a MIC, an amidate, and a phenoxy donor. The structures of all these complexes were established by single-crystal X-ray diffraction studies. Imidazole derivatives are important heterocycles with enormous medicinal value. The catalytic activities of these new palladium complexes in the green direct C–H arylation of imidazoles with aryl halides were investigated and compared to those delivered from palladium complexes with IMes and triazole-based normal NHC ligands. Among the new complexes, the PEPPSI-type palladium complex with the monodentate triazole-based MIC ligand was found to be a very promising precatalyst which was capable of utilizing electron-deficient aryl chlorides as coupling partners in the reaction.


 Please wait while we load your content...
Please wait while we load your content...