Symmetric CEST-active lanthanide complexes for redox monitoring†
Abstract
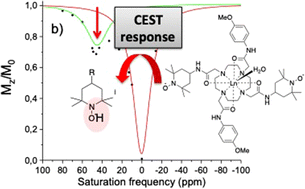
Two symmetric ligands harbouring two TEMPO radicals and two functionalized acetamide arms (R = OMe (L1), CF3 (L2)) were prepared and chelated to lanthanide ions (EuIII, YbIII for both L1 and L2, DyIII for L1). Luminescence measurements on the europium complexes support the coordination of a single water molecule. The TEMPO arms are magnetically interacting in L1 (and its complexes) but not in L2. The TEMPO moieties can be reversibly oxidized into an oxoammonium (0.33–0.36 V vs. Fc+/Fc) or reduced into a hydroxylamine (ill-defined redox wave, reduction by ascorbate), which are both diamagnetic. The europium complexes [Eu(L1)]3+ and [Eu(L2)]3+ in their hydroxylamine form exhibit a temperature dependent CEST effect, which is maximal at 25 °C (30%) and 37 °C (12%), respectively. The CEST activity is dramatically reduced in the corresponding nitroxide forms due to the paramagnetism of the ligand. The europium complexes show no cytotoxicity against M21 cell lines over long incubation times (72 h) at high concentration (40 μM).
- This article is part of the themed collection: New Talent: Europe


 Please wait while we load your content...
Please wait while we load your content...