Palladium(ii) ortho-cyano-aminothiophenolate (ocap) complexes†
Abstract
A series of Pd(II) complexes containing ortho-cyano-aminothiophenolate (ocap) ligands have been prepared and their molecular structures elucidated. Hg(II) ocap complexes, [Hg{SC6H3XN(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N)}]n (X = H, Me) (1), react with Na2S to afford HgS and Na2[ocap] which reacts in situ with K2[PdCl4] to afford palladium ocap complexes [Pd{SC6H3XN(C
N)}]n (X = H, Me) (1), react with Na2S to afford HgS and Na2[ocap] which reacts in situ with K2[PdCl4] to afford palladium ocap complexes [Pd{SC6H3XN(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N)}]n (2). A second route to these coordination polymers has also been developed from reactions of 2-aminobenzothiazole (abt) complexes, trans-PdCl2(abt)2 (3), with NaOH. We have not been able to crystallographically characterise coordination polymers 2, but addition of PPh3, a range of phosphines and cyclic diamines affords mono and binuclear complexes in which the ocap ligand adopts different coordination geometries. With PPh3, binuclear [Pd(μ-κ2,κ1-ocap)(PPh3)]2 (4) results, in which the ocap bridges the Pd2 centre acting as an S,N-chelate to one metal centre and binding the second via coordination of the cyanide nitrogen. In contrast, with diphosphines, Ph2P(CH2)nPPh2 (n = 1–4), mononuclear species predominate as shown in the molecular structures of Pd(κ2-ocap){κ2-Ph2P(CH2)nPPh2} (5–7; n = 1–3). With 2,2′-bipy and 1,10-phen we propose that related monomeric chelates Pd(κ2-ocap)(κ2-bipy) (9) and Pd(κ2-ocap)(κ2-phen) (10) result but we have been unable to substantiate this crystallographically. Addition of HgCl2(phen) to 9a (generated in situ) affords heterobimetallic Pd(κ2-phen)(μ-κ2,κ1-ocap)HgCl2(κ2-phen) (11), in which Hg(II) is coordinated through the ring sulfur.
N)}]n (2). A second route to these coordination polymers has also been developed from reactions of 2-aminobenzothiazole (abt) complexes, trans-PdCl2(abt)2 (3), with NaOH. We have not been able to crystallographically characterise coordination polymers 2, but addition of PPh3, a range of phosphines and cyclic diamines affords mono and binuclear complexes in which the ocap ligand adopts different coordination geometries. With PPh3, binuclear [Pd(μ-κ2,κ1-ocap)(PPh3)]2 (4) results, in which the ocap bridges the Pd2 centre acting as an S,N-chelate to one metal centre and binding the second via coordination of the cyanide nitrogen. In contrast, with diphosphines, Ph2P(CH2)nPPh2 (n = 1–4), mononuclear species predominate as shown in the molecular structures of Pd(κ2-ocap){κ2-Ph2P(CH2)nPPh2} (5–7; n = 1–3). With 2,2′-bipy and 1,10-phen we propose that related monomeric chelates Pd(κ2-ocap)(κ2-bipy) (9) and Pd(κ2-ocap)(κ2-phen) (10) result but we have been unable to substantiate this crystallographically. Addition of HgCl2(phen) to 9a (generated in situ) affords heterobimetallic Pd(κ2-phen)(μ-κ2,κ1-ocap)HgCl2(κ2-phen) (11), in which Hg(II) is coordinated through the ring sulfur.
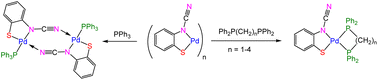


 Please wait while we load your content...
Please wait while we load your content...