Tuning ESIPT-coupled luminescence by expanding π-conjugation of a proton acceptor moiety in ESIPT-capable zinc(ii) complexes with 1-hydroxy-1H-imidazole-based ligands†
Abstract
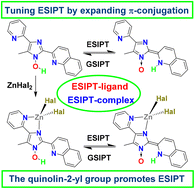
The emission of ESIPT-fluorophores is known to be sensitive to various external and internal stimuli and can be fine-tuned through substitution in the proton-donating and proton-accepting groups. The incorporation of metal ions in the molecules of ESIPT fluorophores without their deprotonation is an emerging area of research in coordination chemistry which provides chemists with a new factor affecting the ESIPT reaction and ESIPT-coupled luminescence. In this paper we present 1-hydroxy-5-methyl-4-(pyridin-2-yl)-2-(quinolin-2-yl)-1H-imidazole (HLq) as a new ESIPT-capable ligand. Due to the spatial separation of metal binding and ESIPT sites this ligand can coordinate metal ions without being deprotonated. The reactions of ZnHal2 with HLq afford ESIPT-capable [Zn(HLq)Hal2] (Hal = Cl, Br, I) complexes. In the solid state HLq and [Zn(HLq)Hal2] luminesce in the orange region (λmax = 600–650 nm). The coordination of HLq by Zn2+ ions leads to the increase in the photoluminescence quantum yield due to the chelation-enhanced fluorescence effect. The ESIPT process is barrierless in the S1 state, leading to the only possible fluorescence channel in the tautomeric form (T), S1T → S0T. The emission of [Zn(HLq)Hal2] in the solid state is blue-shifted as compared with HLq due to the stabilization of the ground state and destabilization of the excited state. In CH2Cl2 solutions, the compounds demonstrate dual emission in the UV (λmax = 358 nm) and green (λmax = 530 nm) regions. This dual emission is associated with two radiative deactivation channels in the normal (N) and tautomeric (T) forms, S1N → S0N and S1T → S0T, originating from two minima on the excited state potential energy surfaces. High energy barriers for the GSIPT process allow the trapping of molecules in the minimum of the tautomeric form, S0T, resulting in the possibility of the S0T → S1T photoexcitation and extraordinarily small Stokes shifts in the solid state. Finally, the π-system of quinolin-2-yl group facilitates the delocalization of the positive charge in the proton-accepting part of the molecule and promotes the ESIPT reaction.


 Please wait while we load your content...
Please wait while we load your content...