Improved emission of Yb(iii) ions in triazacyclononane-based macrocyclic ligands compared to cyclen-based ones†
Abstract
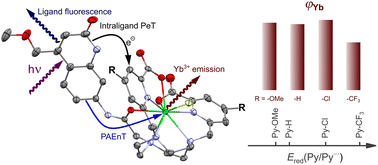
Yb(III) complexes based on ligands with a 1,4,7-triazacyclononane (tacn) macrocyclic core were synthesised. The complexes carry a 4-methoxymethyl-substituted carbostyril chromophore that serves as a light-harvesting antenna. The ligands supply 5 nitrogen and 3 oxygen donors via 1 methylenecarboxamide and 2 picolinate donors, creating +1 charged complexes with an octadentate binding environment. The electronic properties of the picolinates are modulated by varying the substitution at the 4 position with OMe, H, Cl, or CF3. Cyclic voltammetry indicated that the tacn-based Yb(III) complexes were easier to reduce than the analogous cyclen complexes. The first reductive event is likely picolinate-centred, followed by the formation of further reduced species. Antenna excitation yielded Yb(III) luminescence in the near-infrared (NIR) region in all cases. The antenna photophysical properties were consistent with intraligand photoinduced electron transfer from the excited carbostyril to the picolinate groups. The relative quantum yields of Yb(III) luminescence were determined. The lowest value was obtained for the complex with the most efficient antenna-to-picolinate photoinduced electron transfer. Despite intraligand electron transfer quenching of the antenna, the tacn-based Yb complexes were more emissive than their cyclen analogues, highlighting the influence of the ligand structure on the luminescence properties of NIR emissive lanthanide(III) ions.
- This article is part of the themed collection: Nordic Collection


 Please wait while we load your content...
Please wait while we load your content...