Single and double deprotonation/dearomatization of the N,S-donor pyridinophane ligand in ruthenium complexes†
Abstract
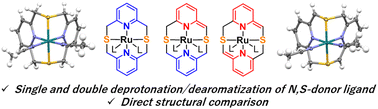
We report a series of ruthenium complexes with a tetradentate N,S-donor ligand, 2,11-dithia[3.3](2,6)pyridinophane (N2S2), that undergo single and double deprotonation in the presence of a base leading to the deprotonation of one or both pyridine rings. Both singly and doubly deprotonated complexes were structurally characterized by single-crystal X-ray diffraction. The NMR spectra are indicative of the dearomatization of one or both pyridine rings upon the deprotonation of the CH2–S arm, similar to the dearomatization of phosphine-containing pincer ligands. The deprotonated (N2S2)Ru complexes did not show appreciable catalytic or stoichiometric reactivity in transfer hydrogenation, hydrogenation and dehydrogenation of alcohols, and attempted activation of H2, CO2, and other substrates. Such a lack of reactivity is likely due to the low stability of the deprotonated species as evident from the structural characterization of one of the decomposition products in which shrinkage of the macrocyclic ring occurs via picolyl arm migration.


 Please wait while we load your content...
Please wait while we load your content...