Quaternary Ru(ii) complexes of terpyridines, saccharin and 1,2-azoles: effect of substituents on molecular structure, speciation, photoactivity, and photocytotoxicity†
Abstract
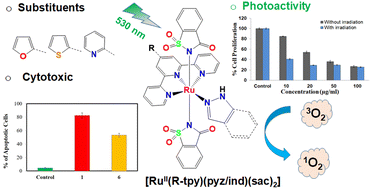
Six photoactive ruthenium quaternary complexes (a four-component system consisting of three different N-donor ligands and Ru(II)): trans-[Ru(R-tpy)(pyz/ind)(sac)2] (1–6) containing substituted terpyridine (R-tpy), saccharin (sac), and monodentate N-donor heterocycles were designed. Here, R-tpy = 4′-(2-furyl (1, 2); thienyl (3, 4); pyridyl (5, 6))-2,2′:6′,2′′ terpyridines, pyz = 1H-pyrazole for 1, 3 and 5 and ind = 1H-indazole for 2, 4 and 6. The azoles are present in a large number of FDA-approved clinical drugs and bioactive molecules. The saccharin acting as a carbonic anhydrase inhibitor (CA-IX) could potentially target aggressive hypoxic tumors that overexpress CA-IX. Such multi-functional ligands bound to a Ru(II)-photocage provide ample scope to tune the electronic structures, photochemistry, and synergistic effect of the photolabile ligands in photoactivated chemotherapy (PACT). The complexes were characterized using various spectroscopic studies, and the molecular structures were determined from X-ray crystallography. They exhibit a distorted octahedral {RuN6} geometry with equatorial sites coordinated to the tridentate N3-donor R-tpy and N-donor pyz/ind, while two transoidal axial sites bound to the N-donor saccharinate (sac) ligands. The solvolysis kinetics showed these complexes undergo facile ligand-exchange reactions in equilibrium with varying rates reflecting the possible electronic effect of the R-groups in R-tpy. The photoreactivity of the complexes in green (λex = 530 nm) LED light indicates that the complexes undergo photodissociation of the monodentate N-donors (i.e., sac/pyz/ind) and showed an efficient generation of singlet oxygen (Φ1O2 = 0.29–0.47), signifying the potential of these complexes in PACT and/or PDT. All the complexes show good binding affinity with CT-DNA with possible intercalation from extended planar polypyridyl ligands with duplex DNA and BSA. The synchronous fluorescence study with BSA suggested preferential interaction at the tryptophan residue in the protein microenvironment. The confocal microscopy studies showed adequate permeability and localization in the cytosol and nucleus of cervical cancer (HeLa) and breast cancer (MCF7) cells. The dose-dependent cytotoxicity of the complexes for both HeLa and MCF7 cells increases upon low-energy (365 nm) photoirradiation. The mechanistic studies revealed that the complexes induce apoptosis and generate reactive oxygen species (ROS) upon green light (λex = 530 nm) irradiation. Overall, these quaternary Ru(II) complexes equipped with three different types of ligands with distinct roles could pave the way for designing multi-targeted chemotherapeutic metallodrugs with synergistic roles for each bioactive ligand.


 Please wait while we load your content...
Please wait while we load your content...