Mixed-valence {Fe II2Fe III4} hexanuclear complexes with thermally induced Fe(iii) spin-crossover behavior†
Abstract
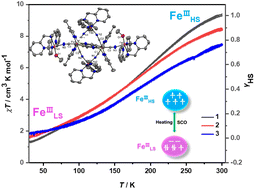
Cyano-bridged mixed-valence {Fe6} hexanuclear complexes {[Tp4-MeFeII(CN)3]2[FeIII(Tpa)]2[FeIII(OR)(Tpa)]2}·6ClO4·S (Tp4-Me = tri(4-methyl-pyrazol-1-yl)borate, Tpa = tris(2-pyridylmethyl)amine; R = –CH3, S = 8MeOH 1, R = –C2H5, S = 6EtOH 2) have been obtained by building the units of [Tp4-MeFe(CN)3]− and [Fe(Tpa)]2+ in methanol and ethanol, respectively. Complex 1 exhibited single-crystal-to-single-crystal (SCSC) transformation to give {[Tp4-MeFeII(CN)3]2[FeIII(Tpa)]2[FeIII(OH)(Tpa)]2}·6ClO4·8H2O (3) in water. Detailed analysis of the coordination environment of the FeIII centers on the square lattice and magnetic susceptibility measurements of all the complexes confirmed their Fe3+ spin-crossover (SCO) properties (T1/2 = 178 K, 1; 185 K, 2; 208 K, 3).


 Please wait while we load your content...
Please wait while we load your content...