Pb3SBrI3: the first Pb-based chalcohalide with multiple halogens features a unique two-dimensional structure composed of diverse Pb-centered polyhedra†
Abstract
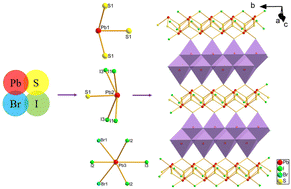
Chalcohalides are attractive multifunctional candidates for new material due to their diverse crystal structures and the characteristics that come from the fusing of chalcogenides and halides. Here, we successfully synthesized a new Pb-based chalcohalide with multiple-halogens, namely, Pb3SBrI3, via a hydrothermal reaction using an organic chemical as the source of the sulfur. The Pb3SBrI3 exhibits unique neutral [Pb3SBrI3]∞ layers composed of rich Pb-centered units including PbS3 trigonal pyramids, PbSI4 square pyramids, and PbBr2I4 octahedra that are piled along the a-axis. Notably, the Pb3SBrI3 is the first Pb-based chalcohalide featuring such a 2D structure. In this work, we examined in detail its synthesis, crystal structure, optical properties and theoretical calculation results.


 Please wait while we load your content...
Please wait while we load your content...