γ-FeO(OH) with multiple surface terminations: Intrinsically active for the electrocatalytic oxygen evolution reaction†
Abstract
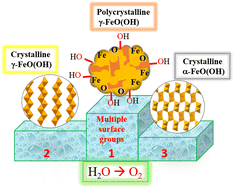
Due to poor conductivity, the electrocatalytic performance of independently prepared iron oxy-hydroxide (FeO(OH)) is inferior whereas FeO(OH) derived in situ from the iron based electro(pre)catalyst shows superior performance in the oxygen evolution reaction (OER). Use of mixed phase FeO(OH) and/or incorporation of CoII/NiII metal into the FeO(OH) structure has also been demonstrated as a convenient approach to achieve high OER activity. Nevertheless, preparation of phase-pure, albeit active FeO(OH) material with fair electrochemical performance remains a perdurable challenge. Moreover, the role of the crystalline phase and its surface structure in controlling the OER activity is still unclear. Herein, a simple synthetic protocol has been developed to prepare a series of phase-pure α-FeO(OH) (goethite) and γ-FeO(OH) (lepidocrocite) materials. By changing the reaction conditions such as iron salt and reaction temperature, the crystallinity as well as the phase of the oxy-hydroxide material have been varied. The isolated α- and γ-FeO(OH) materials with different crystallinity were thereafter deposited on nickel foam (NF) for alkaline OER study. The recorded overpotential value at 10 mA cm−2 has been found to be dependent on the phase and crystallinity of the FeO(OH) materials. The partially crystalline γ-FeO(OH) isolated at room temperature (γ-FeO(OH)@RT) turns out to be the most active with a lowest overpotential of 260 mV at 10 mA cm−2 and a long term stability of 12 h. The γ-FeO(OH)@RT/NF anode can furnish high current densities like 50–100 mA cm−2 which makes this anode distinct from the previously reported FeO(OH) materials. Detailed electrochemical study suggested that the fair activity of the γ-FeO(OH)@RT arises due to a facile electrokinetics as evident from the small Tafel slope and charge transfer resistance (Rct value from the Nyquist plot). Owing to the superior activity of the γ-FeO(OH)@RT/NF, the anode can further be incorporated into an overall water splitting electrolyzer that can operate at a cell potential of 1.68 V. The microscopic characterization provides concrete evidence in support of the polycrystallinity of the γ-FeO(OH)@RT. The superior activity of the γ-FeO(OH)@RT perhaps can be correlated to its polycrystalline nature with more defect edges, the presence of a large exposed surface and random atomic arrangements. The highest degree of multiple surface active terminals (–O, –OH and –Fe) available in this polycrystalline γ-FeO(OH) perhaps makes the catalyst more active compared to the crystalline FeO(OH) analogue with a limited number of surface terminals. From a comparative study with a series of FeO(OH) materials, this work highlights a direct relationship between the surface functionality and the electrochemical activity of the FeO(OH) material.


 Please wait while we load your content...
Please wait while we load your content...