Dual-platination and induced oxidation of uridine by a photoactivatable diazido Pt(iv) anticancer prodrug†
Abstract
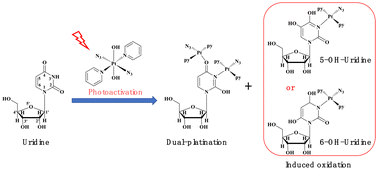
Photoactivatable Pt(IV) anticancer prodrug trans,trans,trans-[Pt(N3)2(OH)2(pyridine)2] (1) has been shown to bind to and induce oxidation of all four DNA nucleobases. Herein, to further render the binding spectrum of complex 1 to nucleic acids, the interaction between complex 1 and uridine, an exclusive RNA component, was investigated by electrospray ionization mass spectrometry (ESI-MS) and NMR spectroscopy. The results showed that complex 1 can bind to uridine through the N3 (major) and O4 (minor) sites upon light irradiation to form the major mono-platinated uridine adduct and the minor di-platinated uridine adduct. Moreover, mono-platinated uridine associated with the oxidation of uridine to 5-hydroxyuridine and 6-hydroxyuridine was also observed. This is the first report that the photoactivatable Pt(IV) prodrug binds to and induces the oxidation of uridine, and also the last piece of the puzzle for the interactions of complex 1 with nucleobases. Combined with our previous results about the interactions between complex 1 and DNA bases, these data showed a wide interaction spectrum of this kind of photoactivatable diazido Pt(IV) prodrugs with nucleobases through such dual-action modes, strongly suggesting that RNA may be a potential target of Pt(IV) prodrugs.


 Please wait while we load your content...
Please wait while we load your content...