Abstract
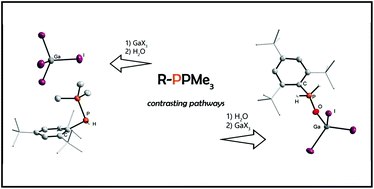
Phosphanylidenephosphoranes of the type R–P(PR′3), also known as phospha-Wittig reagents, can be utilized in a variety of bond activation reactions exploiting their phosphinidenoid reactivity. Herein, we thoroughly show that a facile PMe3 for H2O exchange gives access to various primary phosphine oxides of the general formula RP(H)2O (R = Mes*, MesTer, DipTer) and the molecular structure of DipTerP(O)H2 was determined. However, phosphanylidenephosphoranes are described to be highly nucleophilic as well. We show that the attachment of main group Lewis acids such as GaCl3 and GaI3 to R–P(PMe3) yielded the highly sensitive, yet stable coordination compounds [(RPGaX3)PMe3] (R = Mes*, DipTer) or [(RPPMe3)2GaCl2]GaCl4 (R = MesTer). In contrast to the free phosphanylidenephosphoranes, these species reacted differently with H2O, which was demonstrated for [(Mes*PPMe3)GaI3]. Here the formation of the phosphino-phosphonium cation [Mes*P(H)PMe3]+ and different anions was observed with combined NMR spectroscopic and SC-XRD (SC-XRD = single crystal X-ray diffraction analysis) studies. This work demonstrates that the ambiphilic character of phosphanylidenephosphoranes can be utilized to manipulate the reactivity of R–P(PMe3) towards water, giving primary phosphine oxides, whereas the Lewis acid adducts [(RPGaX3)PMe3] gave phosphino-phosphonium species.


 Please wait while we load your content...
Please wait while we load your content...