Electronic insights into aminoquinoline-based PNHN ligands: protonation state dictates geometry while coordination environment dictates N–H acidity and bond strength†
Abstract
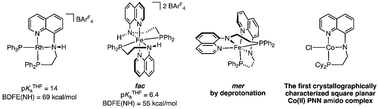
A variety of transition metal complexes bearing aminoquinoline PNHH′-R ligands R = Ph (L1H), Cy (L2H) and their amido analogues are reported for rhodium(I) ([Rh(L1H)(PPh3)]+1 and Rh(L1)(PPh3) 2), cobalt(II) (Co(L2)(Cl) 3), and iron(II) ([Fe(L1H)2]2+5, Fe(L1)26, and [Fe(C5Me5)(L1H)]PF67). The acid–base and redox properties of the amido complexes 2, 6, and their protio parent complexes 1, and 5 permit the determination of the pKa and bond dissociation free energy (BDFE) of their N–H bonds while the ligand scaffold is coordinated to metal centres of square planar and octahedral geometry, respectively. From relative concentrations obtained by the use of 31P{1H} NMR spectroscopy, a pKaTHF value of 14 is calculated for rhodium complex 1, 6.4 for iron complex 5, and 24 for iron complex 7. These data, when combined with elecrochemical potentials obtained via cyclic voltammetry, allow the calculations of BDFE values for the N–H bond of 69 kcal mol−1 for 1, and of 55 kcal mol−1 for 5.


 Please wait while we load your content...
Please wait while we load your content...