Non-heme oxoiron complexes as active intermediates in the water oxidation process with hydrogen/oxygen atom transfer reactions†
Abstract
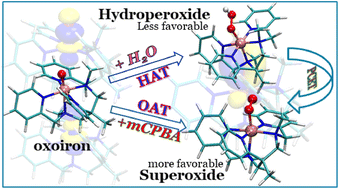
In this study, we explore the water oxidation process with the help of density functional theory. The formation of an oxygen–oxygen bond is crucial in the water oxidation process. Here, we report the formation of the oxygen–oxygen bond by the N5-coordinate oxoiron species with a higher oxidation state of FeIV and FeV. This bond formation is studied through the nucleophilic addition of water molecules and the transfer of the oxygen atom from meta-chloroperbenzoic acid (mCPBA). Our study reveals that the oxygen–oxygen bond formation by reacting mCPBA with FeV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O requires less activation barrier (13.7 kcal mol−1) than the other three pathways. This bond formation by the oxygen atom transfer (OAT) pathway is more favorable than that achieved by the hydrogen atom transfer (HAT) pathway. In both cases, the oxygen–oxygen bond formation occurs by interacting the σ*dz2–2pz molecular orbital of the iron-oxo intermediate with the 2px orbital of the oxygen atom. From this study, we understand that the oxygen–oxygen bond formation by FeIV
O requires less activation barrier (13.7 kcal mol−1) than the other three pathways. This bond formation by the oxygen atom transfer (OAT) pathway is more favorable than that achieved by the hydrogen atom transfer (HAT) pathway. In both cases, the oxygen–oxygen bond formation occurs by interacting the σ*dz2–2pz molecular orbital of the iron-oxo intermediate with the 2px orbital of the oxygen atom. From this study, we understand that the oxygen–oxygen bond formation by FeIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O with the OAT process is also feasible (16 kcal mol−1), suggesting that FeV
O with the OAT process is also feasible (16 kcal mol−1), suggesting that FeV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O may not always be required for the water oxidation process by non-heme N5-oxoiron. After the oxygen–oxygen bond formation, the release of the dioxygen molecule occurs with the addition of the water molecule. The release of dioxygen requires a barrier of 7.0 kcal mol−1. The oxygen–oxygen bond formation is found to be the rate-determining step.
O may not always be required for the water oxidation process by non-heme N5-oxoiron. After the oxygen–oxygen bond formation, the release of the dioxygen molecule occurs with the addition of the water molecule. The release of dioxygen requires a barrier of 7.0 kcal mol−1. The oxygen–oxygen bond formation is found to be the rate-determining step.


 Please wait while we load your content...
Please wait while we load your content...