Synthesis and single crystal X-ray study of phenylselenyl embedded coumarin-based sensors for selective detection of superoxide†
Abstract
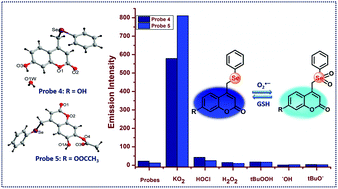
Selenium-coumarin based probe 4 was synthesized from the reaction of a bromo derivative of coumarin with in situ prepared sodium phenyl selenide. Esterification of probe 4 resulted in the formation of probe 5. In the single crystal X-ray structure of probe 4, the phenylselenide group was placed parallel to the coumarin moiety whereas in probe 5, the phenylselenide group was perpendicular to the coumarin group. Hydrogen bonding interactions have been observed in the crystal packing of both the probes. Both the probes selectively detect superoxide over other reactive oxygen species (ROS). A kinetic study of the probes was performed, which suggested that probe 4 achieved maximum fluorescence intensity in less than 1 s while probe 5 required 30 min to attain maximum fluorescence intensity. Both probes 4 and 5 showed 37 fold and 48 fold increase in fluorescence intensity after the reaction with superoxide, respectively. The detection limits of probes 4 and 5 were found to be 46.4 nM and 48.9 nM, respectively. Both the probes showed reversibility with biothiols such as glutathione (GSH) and N-acetyl-L-cysteine (NAC). Probe 5 underwent more redox cycles with GSH compared to probe 4.


 Please wait while we load your content...
Please wait while we load your content...