Ni(ii) complexes of a new tetradentate NN′N′′O picolinoyl-1,2-phenylenediamide-phenolate redox-active ligand at different redox levels†‡
Abstract
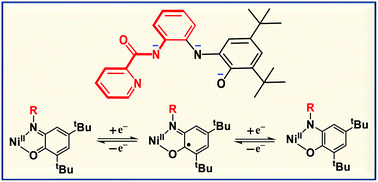
Three square planar nickel(II) complexes of a new asymmetric tetradentate redox-active ligand H3L2 in its deprotonated form, at three redox levels, open-shell semiquinonate(1−) π radical, quinone(0) and closed-shell dianion of its 2-aminophenolate part, have been synthesized. The coordinated ligand provides N (pyridine) and N′ and N′′ (carboxamide and 1,2-phenylenediamide, respectively) and O (phenolate) donor sites. Cyclic voltammetry on the parent complex [Ni(L2)] 1 in CH2Cl2 established a three-membered electron-transfer series (oxidative response at E1/2 = 0.57 V and reductive response at −0.32 V vs. SCE) consisting of neutral, monocationic and monoanionic [Ni(L2)]z (z = 0, 1+ and 1−). Oxidation of 1 with AgSbF6 affords [Ni(L2)](SbF6) (2) and reduction of 1 with cobaltocene yields [Co(η5-C5H5)2][Ni(L2)] (3). The molecular structures of 1·CH3CN, 2·0.5CH2Cl2 and 3·C6H6 have been determined by X-ray crystallography at 100 K. Characterization by 1H NMR, X-band EPR (gav = 2.006 (solid); 2.008 (CH2Cl2–C6H5CH3 glass); 80 K) and UV-VIS-NIR spectral properties established that 1, 2 and 3 have [NiII{(L2)˙2−}], [NiII{(L2)−}]+/1+ and [NiII{(L2)3−}]−/1− electronic states, respectively. Thus, the redox processes are ligand-centred. While 1 possesses paramagnetic St (total spin) = 1/2, 2 and 3 possess diamagnetic ground-state St = 0. Interestingly, the variable-temperature (2–300 K) magnetic measurement reveals that 1 with the St = 1/2 ground state attains the antiferromagnetic St = 0 state at a very low temperature, due to weak noncovalent interactions via π–π stacking. Density functional theory (DFT) electronic structural calculations at the B3LYP level of theory rationalized the experimental results. In the UV-VIS-NIR spectra, broad absorptions are recorded for 1 and 2 in the range of 800–1600 nm; however, such an absorption is absent for 3. Time-dependent (TD)-DFT calculations provide a very good fit with the experimental spectra and allow us to identify the observed electronic transitions.


 Please wait while we load your content...
Please wait while we load your content...