Supramolecular isomorphic dodecanuclear cobalt clusters with the same metal shell but different core ligands†
Abstract
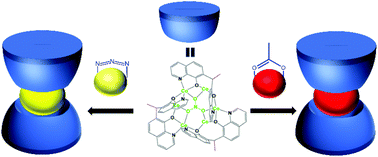
In this work, we report two supramolecular isomorphic dodecanuclear cobalt complexes, [Co12(mtz)3(L)6(NO3)2(OH)(N3)3]·(OH)3 (1) and [Co12(mtz)3(L)6(NO3)2(OH)(N3)(OAc)]·(OH)4 (2), (Hmtz = 5-methyl-1H-tetrazole, H2L = 7,7′-(ethane-1,1-diyl) diquinolin-8-ol) crystallizing in the ![[P with combining macron]](https://www.rsc.org/images/entities/i_char_0050_0304.gif) space group with the same unit cell parameters. In 1 and 2, two pirate hat-like hexanuclear Co6(NO3)(L)3 units form the same dodecanuclear metal shell, but the ligands between the hexanuclear units as the core are distinct. The introduction of acetate anions leads to a blue shift of the absorption band in the visible region. Magnetism studies indicate an antiferromagnetic interaction between the CoII ions in the clusters.
space group with the same unit cell parameters. In 1 and 2, two pirate hat-like hexanuclear Co6(NO3)(L)3 units form the same dodecanuclear metal shell, but the ligands between the hexanuclear units as the core are distinct. The introduction of acetate anions leads to a blue shift of the absorption band in the visible region. Magnetism studies indicate an antiferromagnetic interaction between the CoII ions in the clusters.


 Please wait while we load your content...
Please wait while we load your content...