Effects of Ru doping on the structural stability and electrochemical properties of Li2MoO3 cathode materials for Li-ion batteries†
Abstract
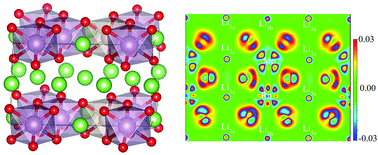
The Li2MoO3 (LMO) material is one promising cathode material for lithium-ion batteries due to its high specific capacity and absence of oxygen release. However, its surface instability in air and poor conductivity have limited its application. To solve these problems, the Ru element has been successfully introduced into the LMO lattice with the aid of the molten salt method. XRD and TEM characterization showed that the introduction of Ru does not change the crystal structure but expands the crystal plane spacing of the {001} facets, which is further evidenced by density functional theory (DFT) calculations. XPS and EDS tests indicated that the introduction of Ru inhibits the oxidation of Mo species and leads to a more uniform distribution of the material. In addition, DFT calculations revealed that covalent interactions are formed between Mo4d/Ru4d and O2p orbitals, leading to a significant reduction of the band gap. Therefore, Ru-doped samples exhibit good electrochemical performances. The initial discharge capacity of an LMRO-2 sample reaches 299.1 mA h g−1 at a 1C rate, and the capacity remains at 125.2 mA h g−1 after 100 cycles. In comparison, the initial discharge capacity of pure phase sample LMO is only 250.5 mA h g−1 under the same conditions, and the capacity remains only at 76.5 mA h g−1 after 100 cycles. The present results confirmed that Ru doping is an effective strategy to improve the performance of the LMO cathode material.


 Please wait while we load your content...
Please wait while we load your content...