NASICON-structured Na3Fe2PO4(SO4)2: a potential cathode material for rechargeable sodium-ion batteries
Abstract
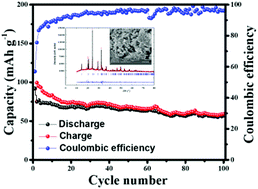
The cost-effective and abundant availability of sodium offers an opportunity for rechargeable Na-ion batteries as an ideal replacement for rechargeable Li-ion batteries. However, the larger size and strong Na+–Na+ interaction create multidimensional phase instability and transformation problems, especially in layer-structured NaxMO2 (Mn, Co, Fe, and Ni) that inhibit the direct transformation of rechargeable Li-ion battery technology to Na-ion batteries. However, framework structures offer superior structural stability due to the interconnection of polyanions or polyhedra forming cationic octahedra. Sodium superionic conductor (NASICON)-type structures are well known for their superior Na+ ion transport and are identified as intercalative hosts as electrodes for rechargeable Na-ion batteries. Here, we report the synthesis of Na3Fe2PO4(SO4)2 in a NASICON framework structure and its investigation as a cathode in a Na/Na3Fe2PO4(SO4)2 cell working on the Fe3+/Fe2+ redox couple. The cell provides a single-phase reaction having a capacity approaching 70 mA h g−1 at 0.1 C after 50 cycles in the voltage range of 2 to 4.2 V, with a columbic efficiency approaching 100%. The large availability of Na and Fe with the stable redox and charge/discharge performance of NASICON-type Na3Fe2PO4(SO4)2 make it a possible cathode candidate for next-generation rechargeable sodium-ion batteries.


 Please wait while we load your content...
Please wait while we load your content...