Host-guest chemistry of a bidentate silyl-triflate bis-Lewis acid – complex complexation behaviour unravelled by diffusion NMR spectroscopy†
Abstract
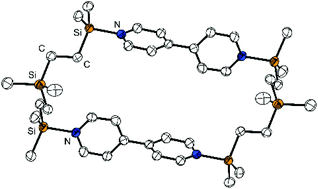
The bidentate silicon-based Lewis acid, bis(dimethyl-(trifluoromethylsulfonyl)silylethyl)dimethylsilane, Me2Si[(CH2)2SiMe2OTf]2, was prepared in a two-step synthesis starting from dimethyldivinylsilane by hydrosilylation with dimethylchlorosilane and subsequent Lewis acidity enhancement of the terminal silicon atoms by substituting the chlorine with triflate groups using silver triflate. The potential of the resulting Me2Si[(CH2)2SiMe2OTf]2 for binding of Lewis basic guests was explored in reactions with mono- and bifunctional aromatic nitrogen bases. A 1 : 2-adduct with pyridine and a 2 : 2-adduct with 4,4′-bipyridine was structurally characterised in the solid state. In solution, diffusion NMR spectroscopy revealed the existence of complex dynamic equilibria of oligomers which are formed by the host with bidentate guests. The size of the oligomers is significantly determined by the spatial arrangement of the docking sites within the guests and depends on the host-guest ratio.


 Please wait while we load your content...
Please wait while we load your content...