Synthesis of the β-dipyrrinyl triphyrin(2.1.1) ligand and its coordination complexes†
Abstract
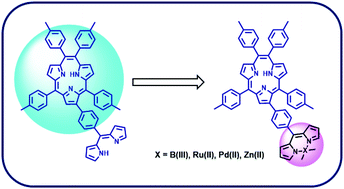
Functionalized β-formylphenyl triphyrin(2.1.1) was prepared by coupling β-bromo triphyrin(2.1.1) with 4-formylphenyl boronic acid under Pd(0) coupling conditions. β-Formylphenyl triphyrin(2.1.1) was treated with excess pyrrole under acid catalyzed conditions in CH2Cl2 to obtain dipyrramethanyl triphyrin(2.1.1), which was subjected to oxidation by treating it with DDQ to obtain the desired ligand, β-dipyrrinyl triphyrin(2.1.1). The dipyrrinyl unit of triphyrin(2.1.1) can act as a bidentate ligand to form interesting coordination complexes. Thus, the dipyrrinyl triphyrin(2.1.1) ligand was treated with BF3·(OEt)2 as well as metal salts such as [Ru(p-cymene)Cl2]2, Pd(acac)2 and Zn(CH3COO)2 to obtain BODIPY-triphyrin(2.1.1), Pd(II)dipyrrin-triphyrin(2.1.1), Ru(II)-dipyrrin-triphyrin(2.1.1) and bis(Zn dipyrrin)-triphyrin(2.1.1) conjugates in good yields. The ligand and all four conjugates were freely soluble in common organic solvents and thoroughly characterized and studied by HR-MS, 1D & 2D NMR spectroscopy, absorption, cyclic voltammetry and DFT/TD-DFT studies. The optimized structures indicated that the triphyrin(2.1.1) and BODIPY/metal dipyrrin units in the conjugates were oriented w.r.t each other with an angle in the range of 25.18°–77.55°. The spectral studies indicated that the two moieties in the conjugates interact weakly and retain their individual characteristics, whereas electrochemical studies revealed their electron deficient nature. The TD-DFT studies were in agreement with the experimental observations.


 Please wait while we load your content...
Please wait while we load your content...