Synthesis and coordination behaviour of 1H-1,2,3-triazole-4,5-dithiolates†
Abstract
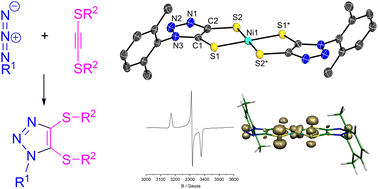
The preparative access to and first group 10 metal complexes of novel 1H-1,2,3-triazole-4,5-dithiolate ligands (tazdt2−) are reported. A set of S-protected 1H-1,2,3-triazole-4,5-dithiol derivatives with R1 = 2,6-dimethylphenyl (Xy) or benzyl (Bn) at N1 and with R2 = Bn or trimethylsilylethyl (TMS-ethyl) at both S atoms were synthesized by a 1,3-dipolar cycloaddition catalysed by either Ru(II) or Cu(I). Extensive investigations on the removal of the protective groups resulted the reductive removal of benzyl groups to be superior in isolating the free 4,5-dithiols of R1N3C2(SH)2 with R1 = Xy (H2-8) or Bn (H2-9). Coordination of these ligands led to the formation of the metal complexes [(η5-C5H5)2Ti(8)], [Ni(dppe)(8)], [Ni(dppe)(9)], [Pd(dppe)(9)] {dppe = bis(diphenylphosphanyl)ethane} and homoleptic (NBu4)n[Ni(8)2] (n = 1, 2). All complexes were fully characterized including structure determination by single crystal XRD. The electronic properties of the Ni and Pd complexes were determined by cyclic voltammetry, UV/vis and EPR spectroscopy supported by DFT calculations. According to the spectral and electrochemical data, the tazdt2− complexes resemble the corresponding benzene-1,2-dithiolate (bdt2−) type compounds reflecting the restricted influence of the electron-withdrawing N3 moiety in the backbone. DSC-TGA measurements with [(η5-C5H5)2Ti(8)] and [Ni(dppe)(8)] indicate a well-defined thermal process involving simultaneous elimination of both N2 and CS.


 Please wait while we load your content...
Please wait while we load your content...