Probing the redox-conversion of Co(ii)-disulfide to Co(iii)-thiolate complexes: the effect of ligand-field strength†
Abstract
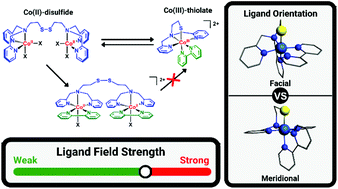
The redox-conversion reaction of cobalt(II)-disulfide to cobalt(III)-thiolate complexes triggered by addition of the bidentate ligand 2,2′-bipyridine has been investigated. Reaction of the cobalt(II)-disulfide complex [Co2(L1SSL1)(X)4] (L1SSL1 = di-2-(bis(2-pyridylmethyl)amino)-ethyldisulfide; X = Cl or Br) [1X] with 2,2′-bipyridine (bpy) resulted in the formation of two different products, namely the cobalt(III)-thiolate complex [Co(L1S)(bpy)]X2 and the unexpected side product [Co2(L1SSL1)(bpy)2(X)2]X2. Crystals of [Co2(L1SSL1)(bpy)2(Cl)2](BPh4)2 [2Cl](BPh4)2 obtained after anion exchange showed the cobalt(II) ions to be in octahedral geometries with the nitrogen donors of the disulfide ligand arranged in a facial conformation and the chloride ion trans to the tertiary amine nitrogen. Remarkably, this side product cannot be converted to the cobalt(III)-thiolate compound [Co(L1S)(bpy)](SbF6)2 [3](SbF6)2 by removal of the chloride ion with use of a silver salt, as this causes scrambling of the ligands, resulting in the formation of [Co(bpy)3]n+. [Co(L1S)(bpy)](SbF6)2 was obtained in a pure form by addition of bpy to a solution in acetonitrile of the compound [Co(L1S)(MeCN)2]2+ [4]2+. Addition of NEt4Cl to [3](SbF6)2 regenerates the cobalt(II)-disulfide complex [1Cl] as confirmed spectroscopically. DFT studies revealed that the conversion from [1Cl] to [3]2+ most likely occurs via the hypothetical intermediate species [2Cl]2+mer, in which the nitrogen atoms of the disulfide ligand are arranged in a meridional conformation. Interestingly, the estimated d-orbital splitting energy of [3]2+ is lower than that of [4]2+, indicating that the ligand-field strength of bpy is lower than anticipated, which hampers clean conversion in the redox-conversion reaction. This study shows that the redox-conversion reaction between cobalt(II)-disulfide and cobalt(III)-thiolate complexes is intricate rather than straightforward.


 Please wait while we load your content...
Please wait while we load your content...