Selective 1,2 addition of polar X–H bonds to the Ga–P double bond of gallaphosphene L(Cl)GaPGaL†
Abstract
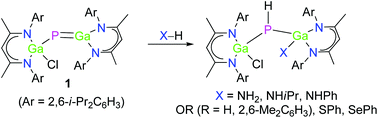
Gallaphosphene L(Cl)GaPGaL 1 (L = HC[C(Me)N(2,6-i-Pr2-C6H3)]2) reacts at ambient temperature with a series of polar X–H bonds, i.e. ammonia, primary amines, water, phenol, thiophenol, and selenophenol, selectively with 1,2 addition at the polar Ga–P double bond. The gallium atom serves as electrophile and the phosphorous atom is protonated in all reactions. The resulting complexes L(Cl)GaP(H)Ga(X)L (X = NH22, NHi-Pr 3, NHPh 4, OH 5, OXyl 6, SPh 7, SePh 8) were characterized by IR and heteronuclear (1H, 13C{1H}, 31P{1H}) NMR spectroscopy, elemental analysis, and single-crystal X-ray diffraction.


 Please wait while we load your content...
Please wait while we load your content...