Luminescent iridium(iii) dipyrrinato complexes: synthesis, X-ray structures, and DFT and photocytotoxicity studies of glycosylated derivatives†
Abstract
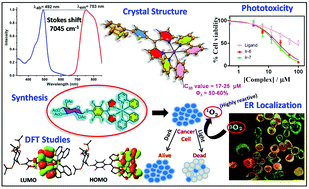
A series of luminescent Ir(III) dipyrrinato complexes were synthesized having various aromatic chromophores at the C-5 position of dipyrrin ligands. The presence of different chromophores on the Ir(III) dipyrrinato complexes altered their optical properties and produced strong emission in the red to NIR region (680–900 nm) with huge Stokes shifts (5910–7045 cm−1). TD-DFT studies indicated significant charge distribution between dipyrrin ligands and Ir-cyclometalated units in all the molecules. X-ray crystal structures revealed an octahedral geometry of the Ir(III) center in the complex. The in vitro studies of the glycosylated Ir(III) complexes revealed strong photoluminescence with maximum Stokes shifts, and they showed significant photocytotoxicity in skin keratinocyte (HaCaT) and lung adenocarcinoma (A549) cells. The singlet oxygen generation quantum yields of glycosylated Ir(III) complexes were in the range of 70–78% in water. The estimated IC50 values were between 17 and 25 μM after light exposure, and confocal microscopy revealed significant localization of the glycosylated Ir(III) complexes in the endoplasmic reticulum (ER) of cancer cells. The neutral Ir(III) dipyrrinato complexes are promising tracking agents for cellular imaging in the biological window and for photodynamic therapy (PDT) applications.


 Please wait while we load your content...
Please wait while we load your content...