Insight into fragmentation of a phosphirane to form phosphinidene complexes: an illustration with the 1-phenylselenylphosphirane W(CO)5 complex†
Abstract
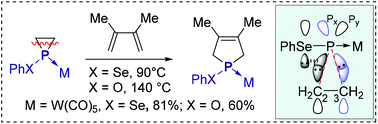
Density functional theory (DFT) calculations with 1-phenylselenylphosphirane complex 1 provide an insight into phosphirane fragmentation to phosphinidene complexes. FMO and ELF analyses show that the cleavage of two P–C σ bonds of phosphirane proceeds via an asynchronous concerted pathway. Transient [PhSeP–W(CO)5] was generated by dissociation of 1 at 90 °C and trapped with different reagents. The 1-phenoxylphosphirane complex undergoes [1 + 2] retroaddition at a comparatively higher temperature which implies that the lone pair of the adjacent atom center of phosphorus plays a major role in phosphirane fragmentation.


 Please wait while we load your content...
Please wait while we load your content...