Complexes of transition metal carbonyl clusters with tin(ii) phthalocyanine in neutral and radical anion states: methods of synthesis, structures and properties†
Abstract
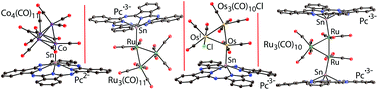
Coordination of tin(II) phthalocyanine to transition metal carbonyl clusters in neutral {SnII(Pc2−)}0 or radical anion {SnII(Pc˙3−)}− states is reported. Direct interaction of Co4(CO)12 with {SnII(Pc2−)}0 yields a crystalline complex {Co4(CO)11·SnII(Pc2−)} (1). There is no charge transfer from the cluster to phthalocyanine in 1, which preserves the diamagnetic Pc2− macrocycle. The Ru3(CO)12 cluster forms complexes with one or two equivalents of {SnII(Pc˙3−)}− to yield crystalline {Cryptand[2.2.2](Na+)}{Ru3(CO)11·SnII(Pc˙3−)}− (2) or {Cryptand[2.2.2](M+)}2{Ru3(CO)10·[SnII(Pc˙3−)]2}2−·4C6H4Cl2 (3) (M+ is K or Cs). Paramagnetic {SnII(Pc˙3−)}− species in 2 are packed in π-stacking [{SnII(Pc˙3−)}−]2 dimers, providing strong antiferromagnetic coupling of spins with exchange interaction J/kB = −19 K. Reduction of Ru3(CO)12, Os3(CO)12 and Ir4(CO)12 clusters by decamethylchromocene (Cp*2Cr) and subsequent oxidation of the reduced species by {SnIVCl2(Pc2−)}0 yield a series of complexes with high-spin Cp*2Cr+ counter cations (S = 3/2): (Cp*2Cr+){Ru3(CO)11·SnII(Pc˙3−)}−·C6H4Cl2 (4), (Cp*2Cr+){Os3(CO)10Cl·SnII(Pc˙3−)}−·C6H4Cl2 (5) and (Cp*2Cr+){Ir4(CO)11·SnII(Pc˙3−)}2− (6). It is seen that reduced clusters are oxidized by SnIV, which is transferred to SnII, whereas the Pc2− macrocycle is reduced to Pc˙3−. In the case of Os3(CO)12, oxidation of the metal atom in the cluster is observed to be accompanied by the formation of Os3(CO)10Cl with one OsI center. Rather weak magnetic coupling is observed between paramagnetic Cp*2Cr+ and {SnII(Pc˙3−)}− species in 4, but this exchange interaction is enhanced in 5 owing to Os3(CO)10Cl clusters with paramagnetic OsI (S = 1/2) also being involved in antiferromagnetic coupling of spins. The formation of {SnII(Pc˙3−)}− with radical trianion Pc˙3− macrocycles in 2–5 is supported by the appearance of new absorption bands in the NIR spectra and essential Nmeso–C bond alternation in Pc (for 3–5). On the whole, this work shows that both diamagnetic {SnII(Pc2−)}0 and paramagnetic {SnII(Pc˙3−)}− ligands substitute carbonyl ligands in the transition metal carbonyl clusters, forming well-soluble paramagnetic solids absorbing light in the visible and NIR ranges.


 Please wait while we load your content...
Please wait while we load your content...