Formation of cyclic (boryl)iminomethane derivatives by the insertion of isocyanides into a boron–carbon bond†
Abstract
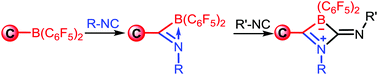
Isocyanides are highly valuable reagents in organic synthesis and have been widely used in multicomponent reactions. Although η2-imidoyl metal complexes, which are important intermediates in isocyanide chemistry, have been extensively explored, their boron species analogues have remained elusive. Hererin, we reported the synthesis of cyclic (boryl)iminomethanes via direct isocyanide insertion into the B–C bond of amino alkenyl boranes in a facile synthetic procedure. A family of well-defined cyclic (boryl)iminomethanes are characterized. Furthermore, the intrinsic ring strain of cyclic (boryl)iminomethanes primes them to further react with isocyanides and selectively afford double insertion products. Our results provide new insights into novel isocyanide chemistry involving boron species.


 Please wait while we load your content...
Please wait while we load your content...