Quadrol-Pd(ii) complexes: phosphine-free precatalysts for the room-temperature Suzuki–Miyaura synthesis of nucleoside analogues in aqueous media†
Abstract
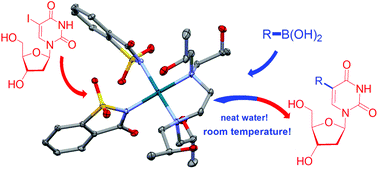
Commercially available Quadrol, N,N,N′,N′-tetrakis(2-hydroxypropyl)ethylenediamine (THPEN), has been used for the first time as a N^N-donor neutral hydrophilic ligand in the synthesis and characterization of new water soluble palladium(II) complexes containing chloride, phthalimidate or saccharinate as co-ligands. [PdCl2(THPEN)] (1) [Pd(phthal)2(THPEN)] (2), [Pd(sacc)2(THPEN)] (3) and the analogous complex with the closely related N,N,N′,N′-tetrakis(2-hydroxyethyl)ethylenediamine (THEEN) [Pd(sacc)2(THEEN)] (4) were efficiently prepared in a one-pot reaction from [PdCl2(CH3CN)2] or Pd(OAc)2. Structural characterization of 1 and 3 by single crystal X-ray diffraction produced the first structures reported to date of palladium complexes with Quadrol. The resultant palladium complexes are highly soluble in water and were found to be effective as phosphine-free catalysts for the synthesis of functionalized nucleoside analogues under room-temperature Suzuki–Miyaura cross-coupling conditions between 5-iodo-2′-deoxyuridine (& 5-iodo-2′-deoxycytidine) with different aryl boronic acids in neat water. This is the first report of the coupling process performed on nucleosides in water at room temperature.


 Please wait while we load your content...
Please wait while we load your content...