Organic solvent-assisted co-precipitation synthesis of red-emitting K2TiF6:Mn phosphors with improved quantum efficiency and optimized morphology†
Abstract
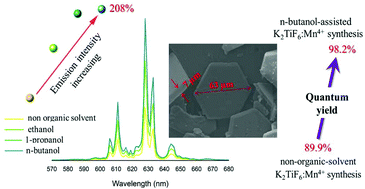
We report an organic solvent–assisted (OSA) co-precipitation strategy for the production of Mn4+-activated K2TiF6 phosphor. The phosphor particle size was controlled through the selection of organic solvents with an alcohol functional group and different carbon chain lengths used in the synthesis. The synergistic effect of the organic solvent and hydrofluoric acid results in large smoothed hexagonal-shaped crystal sheets of particles that become larger as the carbon chain length of the organic solvent increases. The photoluminescence (PL) properties of K2TiF6:Mn powders strongly depend on the size and thickness of the particles. The addition of n-butanol during the synthesis increases the emission intensity of K2TiF6:Mn by 208%. The PL quantum efficiency of phosphors prepared using the n-butanol-assisted strategy is much higher (98.2%) than that of conventionally prepared phosphors (89.9%). Our findings demonstrate a way to prepare the K2TiF6:Mn phosphor with targeted morphology and very high quantum efficiency and also provide the route for the optimization of all Mn4+-activated fluoride phosphors used in white light-emitting diodes.


 Please wait while we load your content...
Please wait while we load your content...