Stable 3D neutral gallium thioantimonate frameworks decorated with transition metal complexes for a tunable photocatalytic hydrogen evolution†
Abstract
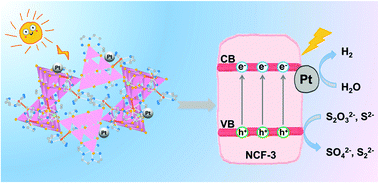
Incorporating transition metal (TM) complexes into cluster-based chalcogenide frameworks is an effective synthetic strategy to induce structural diversity and control the optoelectronic properties, which may further improve their photocatalytic performance. However, limited studies have been conducted on frameworks constructed by TM complexes covalently bonded with supertetrahedral Tn clusters, let alone on their properties, especially photocatalytic H2 activity. Herein, three new isostructural three-dimensional (3D) neutral inorganic–organic open frameworks of gallium thioantimonate comprised of thiogallate-based supertetrahedral T3 clusters that are covalently bonded with TM complexes ([TM(TEPA)]2+, TM = Mn/Ni/Fe, TEPA = tetraethylenepentamine) at the edges and are linked by single Sb3+ ions at the corner, namely, NCF-3-Mn/Ni/Fe have been solvothermally synthesized and structurally characterized, and display good thermal and chemical stability. Benefiting from an adjustable TM centre, the title compounds possess tunable photocatalytic H2 evolution activity, among which NCF-3-Mn exhibits the highest photocatalytic activity probably due to its favourable band structure and enhanced carrier separation efficiency.


 Please wait while we load your content...
Please wait while we load your content...