Enhancement of cancer-cell-selective cytotoxicity by a dicopper complex with a phenanthrene amide-tether ligand conjugate via mitochondrial apoptosis†
Abstract
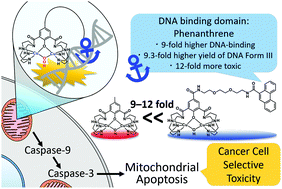
Dicopper complexes [Cu2(μ-OH)(Ln)](ClO4)2 [n = 1 (1) and 2 (2)] with a novel phenanthrene amide-tether ligand conjugate (HL1) and the original p-cresol-2,6-bis(amidecyclen) (HL2) were synthesized. A phenanthrene unit of 1 enhances the DNA-binding by 9-fold, enabling 1 to convert supercoiled plasmid DNA with H2O2 to a linear one in a 9.3-fold higher yield than 2. 1 reacts with H2O2 to form the μ-1,1-hydroperoxodicopper(II) complex 3 as the active species. The IC50 values of 1 against cancer cells of the lung and pancreas are 23.8 and 18.4 μM, respectively, 12-fold more toxic than the 284 and 241 μM of 2. Confocal microscopy, fluorescence-activated cell sorting, and caspase activity assays using HeLa cells revealed that 1 induces mitochondrial apoptosis. A DNA-targeting phenanthrene unit of 1 enhances the cancer-cell-selective toxicity via mitochondrial apoptosis.


 Please wait while we load your content...
Please wait while we load your content...