Structural and mechanistic insights into enantioselectivity toward near-symmetric esters of a novel carboxylesterase RoCE†
Abstract
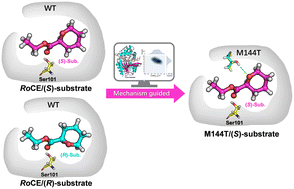
A novel carboxylesterase designated as RoCE was identified from Rhodococcus opacus with high activity and enantioselectivity toward asymmetric esters such as ethyl 2,2-dimethylcyclopropane-1-carboxylate (DMCPE). Moreover, RoCE could catalyze the enantioselective resolution of near-symmetric oxyheterocyclic esters such as ethyl tetrahydro-2H-pyran-2-carboxylate (THPCE), which are generally regarded as “hard-to-be-discriminated” by chemical and biological catalysts. The crystal structure of RoCE was resolved at a resolution of 1.78 Å. Theozyme calculation, MD simulations and pre-reaction state analysis were performed to clarify the molecular basis for the enantioselectivity toward oxyheterocyclic carboxylic acid esters with a nearly symmetric structure. F166 plays an important role in manipulating the enantioselective recognition of (S)- and (R)-DMCPE through steric effect. The intrinsic symmetric structure of (S)- and (R)-THPCE is mainly responsible for the relatively lower enantioselectivity than DMCPE. By introducing hydrogen bond interactions, a mutant M144T was successfully obtained with an E value of 2.44-fold that of WT. MD simulations further prove the increased enantioselectivity of M144T in terms of pre-reaction state and binding free energy. This study provides a novel carboxylesterase and important molecular insights into the enantioselectivity of carboxylesterase toward heterocyclic carboxylic acid esters with a nearly symmetric structure, which will facilitate further engineering of the enantioselectivity of carboxylesterase.


 Please wait while we load your content...
Please wait while we load your content...