Manganese N,N,N-pincer complex-catalyzed epoxidation of unactivated aliphatic olefins†
Abstract
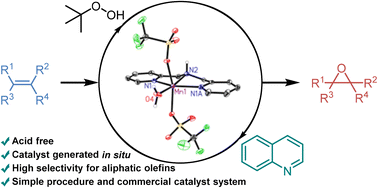
A practical in situ generated manganese(II) catalyst system with the commercially available bis(2-picolyl) amine ligand enables epoxidation of terminal aliphatic olefins in good yields with tert-butyl hydroperoxide as oxidant. Crystallization experiments revealed the formation of two manganese solvent complexes with MeCN and H2O, respectively. Furthermore, a detailed investigation of the quinoline additive identified its crucial role in this transformation as being both essential for the epoxide formation while simultaneously being detrimental to its stability.


 Please wait while we load your content...
Please wait while we load your content...