A concise method for cyclic gem-difluoroacyl scaffolds via visible-light-mediated redox-neutral cascade radical cyclization of alkenes†
Abstract
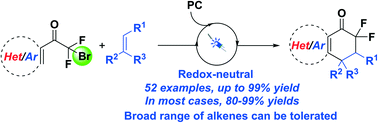
In this paper, a series of diverse alkenes were engaged in radical tandem cyclization initiated by a CF2 radical precursor via photoredox catalysis, affording various cyclic gem-difluoroacyl arenes in good to excellent yields. This photo-induced protocol features a redox-neutral process to access a variety of gem-difluoroacyl arenes with a broad substrate scope and the structural motifs are important in pharmaceutical and agrochemical products. The mechanistic paradigm has been proposed on the basis of control and Stern–Volmer analysis experiments.


 Please wait while we load your content...
Please wait while we load your content...