Valorisation of biobased olefins via Rh-catalyzed transfer hydroformylation and isomerization using formaldehyde as a CO/H2 surrogate†
Abstract
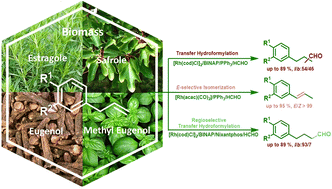
The use of biomass as a new platform of chemical substrates has become a subject of intensive research. In this sense, allylbenzenes and their isomeric analogues represent an important target due to their relevance not only as biological compounds but also as additives for the flavour and food industry. On the other hand, the hydroformylation reaction using syngas surrogates is an attractive tool towards the development of sustainable catalytic methodologies. Herein we report the selective functionalization and isomerization of biomass olefin derivatives, safrole, estragole, eugenol and methyl eugenol, under transfer hydroformylation reaction conditions. In this work, we established that the selection of the rhodium precursor is pivotal for the outcome of the reaction and it would be crucial to promote the in situ formation of syngas. The catalytic system [Rh(cod)Cl]2/rac-BINAP/PPh3 with formaldehyde leads to the selective formation of a mixture of aldehydes, whereas the use of [Rh(acac)(CO)2]/rac-BINAP/PPh3 with formaldehyde quantitatively yields the corresponding isomer derivatives. 1H- and 31P-NMR investigations showed the formation of different Rh-hydride species for each system. Finally, a high linear regioselectivity with the system [Rh(cod)Cl]2/rac-BINAP/nixantphos was achieved.


 Please wait while we load your content...
Please wait while we load your content...